Regulation of Glycemia by Central Nervous System (REGLYS)
The Regulation of Glycemia by the Central Nervous System team focuses on how an organism is able to permanently control its metabolic fluxes in order to maintain glucose homeostasis whatever its energetic status (i.e., fasted or fed). More precisely, the team studies the functioning of three major sites of nutrient detection: the olfactory bulb and the hypothalamus for the central nervous system and the islets of Langerhans for the periphery. Once these nutritional informations are detected and integrated, the team studies the output signals (the autonomic nervous system activity, insulin and glucagon secretion…) which will in turn control the metabolic pathways (catabolic and anabolic) in the skeletal muscles, liver and adipose tissue, finally allowing the maintenance of the glycemia within a physiological range.
The team also integrates in their models the measurement of food intake and feeding behavior. All this work is performed in rodents (rats and mice) under physiological or pathophysiological conditions (preclinical models of obesity or type 2 diabetes, T2D). The team uses normal animals fed standard or high fat/high sucrose diet to induce obesity or T2D. The team also uses obese or diabetic genetic models (Ob/Ob mice, Db/Db, Zucker rats) as well as genetic (Cre-lox, local AAV injection) or chemogenetic (DREADD).

Thematic areas
Role of Nutrients in Inter-Organ Dialogue and Glucose Regulation (Céline Cruciani-Guglielmacci)
Nutrients, particularly lipids, modulate the regulation of glucose homeostasis through their effects on the central nervous system, or “lipid sensing,” and lead to modifications in the neural control of insulin secretion and action, food intake behavior, and energy expenditure. We have highlighted the predominant role of an enzyme responsible for lipid hydrolysis, lipoprotein lipase, in cerebral lipid sensing (Laperrousaz et al., Front Endocrinol 2018; Laperrousaz et al., Diabetologia 2017; Picard et al., Molecular Metabolism 2013). We also contributed to revealing the role of the ACBP (Acyl-CoA binding protein) in the control of food intake and body weight following intravenous administration (Bravo-San Pedro et al., Cell Metab 2009), and we are now exploring the effects of this protein in the olfactory bulb of mice. We have also focused on the Elovl2 enzyme, a long-chain fatty acid elongase. We showed that this enzyme, specific for the endogenous synthesis of docosahexaenoic acid (DHA), was deregulated in vitro under conditions of glucolipotoxicity (when insulin secretion is also decreased), and that restoring its expression via a viral approach led to the restoration of insulin secretion (Bellini et al., Diabetologia 2018; Cruciani-Guglielmacci et al., Mol Metab 2017), through a reduction in ceramide accumulation. We then demonstrated that mice deficient in Serine Palmitoyl Transferase 2 in hepatocytes are protected against obesity induced by a high-fat diet, as well as the resulting glucose intolerance. The mechanisms involved include reduced intestinal lipid absorption due to disruption in bile acid composition, produced by the liver (Lallement et al., BBA lip 2023). In addition to animal models of obesity and type 2 diabetes, we have developed MASH (Metabolic-associated Steatohepatitis) models to test candidate molecules for the treatment of the disease. Finally, we are participating in a study on the role of intestinal gluconeogenesis in the behavioral reinforcement of anorexia. Our hypothesis is that glucose production in the portal vein, stimulated by caloric restriction, could facilitate adaptation to chronic energy deficiency through its effects on the central nervous system.
Inter-organ dialogue
Olfaction and Energy Metabolism Control: Role of Incretins in the Olfactory Bulb (Hirac Gurden)
In a fasting state, our sense of smell is highly sensitive to food odors, allowing us to appreciate what we eat. The first symptom in individuals suffering from olfactory loss (anosmia, hyposmia) is the loss of food enjoyment. In all mammals, the olfactory system not only detects and encodes olfactory information (especially food odors) but also regulates certain mechanisms of energy balance control. A neurometabolic pathway between the olfactory bulb and the pancreas, dependent on GLP-1 signaling, regulates insulin secretion and blood glucose levels (Montaner et al. 2024, Nature Communications).
We have demonstrated in mice the existence of a new neurometabolic axis between the olfactory bulb and the pancreas, which includes a hypothalamic relay via the LHA (lateral hypothalamic area) and the PVN (paraventricular nucleus) that controls both sympathetic and parasympathetic nervous systems (Montaner et al., 2023, Molecular Metabolism; Montaner et al., 2024, Nature Communications). We have shown that this axis is regulated by GLP-1 incretin signaling in the olfactory bulb, which opens the possibility of analyzing the central mechanisms induced by pharmacomimetics of this system, such as Ozempic® or Wegovy® (antidiabetic drugs that induce weight loss), whose brain actions remain poorly understood.
We use murine models of obesity induced by a high-calorie diet (Western Diet), which we compare to age-matched normoweight mice. We continue to study the GLP-1 / GLP-1 Receptor system using pharmacological or pharmacogenetic techniques, as well as its consequences on energy metabolism regulation and olfactory behavior. Finally, we are also studying the outcome of cerebrovascular activity in obese mice (Soleimanzad et al., in press, International Journal of Obesity).
Patient Associations
Hirac Gurden supports the associations “Anosmie.org” (developing an olfactory rehabilitation protocol for individuals suffering from olfactory disorders), “Culture et Hôpital” (creating an olfactory stimulation workshop in geriatrics, EHPAD), and the Miam-Miam group (olfactory goose game for children with oral intake disorders, Hôpital Debré, Paris).
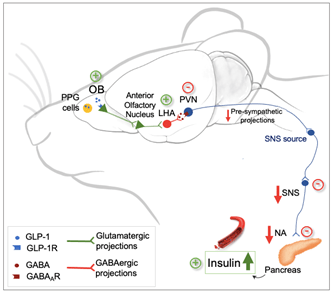
Neurometabolic pathway between the olfactory bulb and the pancreas, dependent on GLP-1 signaling and regulating insulin levels and blood glucose (Montaner et al. 2024, Nature Communications).
Role of Prokineticin in Energy Balance Control (Christophe Magnan)
We are currently studying its effects on food preference in preclinical models of diabetes and obesity, as well as in cohorts of obese and insulin-resistant patients.

Decrypting the links between cognitive deficits and metabolic disorders by modulating the DYRK1A/GSK3 signaling pathways (Nathalie Janel)
The molecular mechanisms of metabolic disorders such as type 2 diabetes and obesity are found to be similar to those of neurodegenerative diseases like Alzheimer’s disease (AD). Therefore, we aim to understand the factors associated with cognitive deficits, diabetes, and obesity for targeted management and prevention strategies. The alteration of certain signaling pathways can be characterized by a brain/pancreas/adipose tissue axis linking beta cell and adipocyte dysfunction to cognitive impairments. We have demonstrated that the DYRK1A kinase is involved not only in deficits in neurogenesis, learning, and memory, but also in inflammation, glucose, and lipid metabolism. To further investigate the role of signaling pathways involving DYRK1A on the brain/pancreas/adipose tissue axis, we will rely on our previous findings demonstrating the positive effects of a molecule with neuroprotective and neurotrophic effects (patent pending) on DYRK1A expression and cognitive disorders.
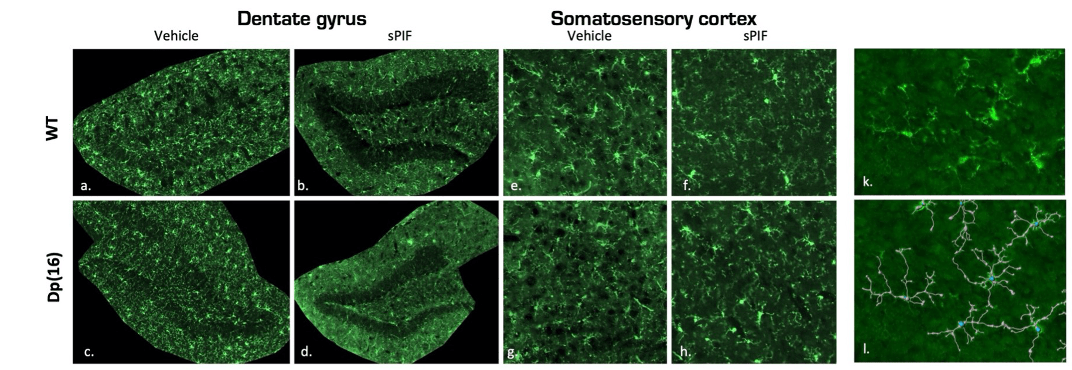
Microglial Branching
Development of Probiotic Products in Diabetes and Obesity (Dominique Gauguier/Christophe Magnan)
Mechanisms associated with improved glucose tolerance following bariatric surgery (Dominique Gauguier)
Understanding ageing through the end-of-life Smurf phenotype (Michaël Rera)
M. Rera has developed a model where ageing is a discontinuous process made of two consecutive phases, each with distinct molecular and physiological properties properties. His group uses this system to study the mechanisms leading the ageing process within this novel framework for studying ageing as a biphasic process using model organisms, addressing the ethical implications of the model and developing innovative approaches for understanding the role played by ageing during evolution.

Smurf phenotype
The Team in Pictures
Mouse Olfactory Bulb (Hirac Gurden)

© BFA, UMR 8251 CNRS – Université Paris Cité
Oil Red O-stained Lipid Droplets in the Liver

© BFA, UMR 8251 CNRS – Université Paris Cité
Schematic Representation of Lipid Detection by the Central Nervous System (Céline Cruciani-Guglielmacci)

© BFA, UMR 8251 CNRS – Université Paris Cité
Recent publications
ABCG1 orchestrates adipose tissue macrophage plasticity and insulin resistance in obesity by rewiring saturated fatty acid pools. Sci Transl Med. 2024 Dec 11;16(777):eadi6682. doi: 10.1126/scitranslmed.adi6682.
Revisited guidelines for metabolic tolerance tests in mice. Lab Anim (NY). 2025 Jan;54(1):16-23. doi: 10.1038/s41684-024-01473-5. Epub 2024 Nov 25.
A multiorgan map of metabolic, signaling, and inflammatory pathways that coordinately control fasting glycemia in mice. iScience. 2024 Oct 11;27(11):111134. doi: 10.1016/j.isci.2024.111134.
A neuronal circuit driven by GLP-1 in the olfactory bulb regulates insulin secretion. Nat Commun. 2024 Aug 13;15(1):6941. doi: 10.1038/s41467-024-51076-4.
Brain lipid sensing and the neural control of energy balance. Biochimie. 2024 Aug;223:159-165. doi: 10.1016/j.biochi.2024.05.020.
Dietary docosahexaenoic acid (DHA) downregulates liver DHA synthesis by inhibiting eicosapentaenoic acid elongation. J Lipid Res. 2024 Jun;65(6):100548. doi: 10.1016/j.jlr.2024.100548. Epub 2024 Apr 20.
Preterm birth: A neuroinflammatory origin for metabolic diseases? Brain Behav Immun Health. 2024 Mar 7;37:100745. doi: 10.1016/j.bbih.2024.100745.
Interesterified palm oil promotes insulin resistance and altered insulin secretion and signaling in Swiss mice. Food Res Int. 2024 Feb;177:113850. doi: 10.1016/j.foodres.2023.113850.
Br J Nutr. 2024 Mar 14;131(5):749-761. doi: 10.1017/S0007114523002404.
Team news
Welcome to Alaa Reguei, new PhD student at TPM2PI.
Alaa Reguei will join the TPM2PI team by october 2025, thank to a grant of thee graduate school "Drug Development". His work will focus on "Conception/optimisation of GPCRs biased modulators, combining AI and molecular simulation".
Call for Applications – BFA
The Functional and Adaptive Biology Unit (BFA, Université Paris Cité, CNRS UMR 8251, INSERM ERL 1133, https://bfa.u-pariscite.fr/ ) is launching a call for applications centered on the integrated study of pathological processes related to the nutritional...
InIdex MetaboBrain
Acceptation du projet InIdex MetaboBrain, auquel l'équipe contribuera.
last opdated: 24/01/2025



