Exploration Physiologique et Fonctionnelle (EPF)
Scientific manager : Serge Luquet (DR CNRS)
Technical manager : Julien Castel (IGE CNRS)
For all requests to use the Metabolism platform, please read the Charte_EPFMetabolismeBFA and return the application form Demande_collaboration_EPFBFA .
© BFA, UMR 8251 CNRS – U Paris Cité
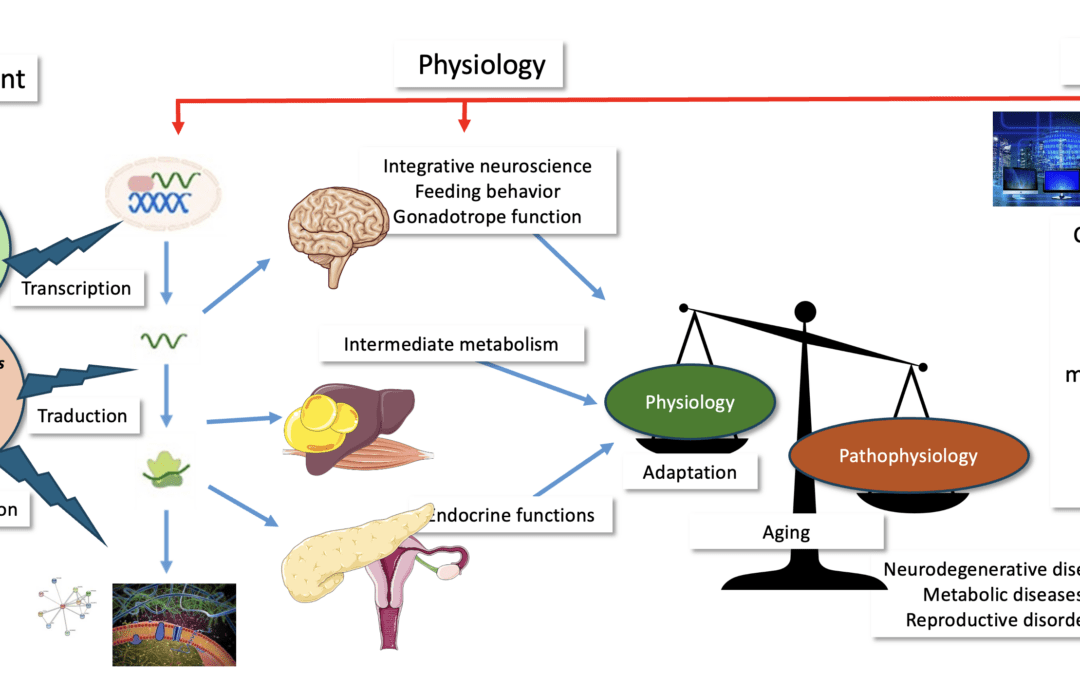
Energy homeostasis is maintained by a complex network of peripheral and central signals which provide information about an organism’s nutritional status and enable it to develop a behavioural and metabolic response to adapt to a change in nutrient availability. Dysregulation of this system leads to the development of pathophysiological disorders, such as anorexia nervosa, or, conversely, obesity-related disorders, such as atherosclerosis, hypertension and type 2 diabetes. To maintain a constant body weight, energy intake from food must be balanced with energy expenditure from physical activity, basal metabolic rate and thermogenesis. An imbalance in this balance can lead to the appearance of the pathophysiological conditions mentioned above. However complex this balance may be, it remains an illustration of the first principle of thermodynamics, which states that energy is neither created nor lost, but is transformed. The EPF philosophy is an application of this principle at the physiological level.
The Physiological and Functional Exploration (EPF) resource enables non-invasive and fully automated analysis of several parameters in mice and rats: food and water intake, food choice, measurement of O₂ consumption and CO₂ production, respiratory quotient (indicative of the nature of glycolytic or oxidative metabolism), overall energy expenditure, as well as spontaneous activity and fine measurement of movements in three dimensions (Phenomaster, TSE Systems GmbH). EPF also enables non-invasive analysis of body composition using NMR technology via an EchoMRI 900 scanner (Echo Medical systems, Houston, USA). This system does not require anaesthesia and can be used to determine lean body mass, fat mass, body fluids and total water composition in mice or rats weighing between 25 and 900 grams. A special modification has been made to EPF to allow chronic perfusion to be accommodated while all the above parameters are simultaneously acquired. This modification offers a unique opportunity to test the effectiveness of pharmacological compounds on energy expenditure and feeding behaviour (guide pratique de calorimétrie).
Since its creation, the EPF resource has been used to provide services at academic (INSERM U872) and private (KOT-CEPRODI) levels, and has led to several academic research collaborations at national and international level (INSERM U845, U855, U939, U837, Institut Cochin, Institut Jacques Monod, IRBHM Brussels, Institut de Biomédecine de Valence, Spain).
Recent publications
2024
- Castel, J., Li, G., Onimus, O., Leishman, E., Cani, P. D., Bradshaw, H., Mackie, K., Everard, A., Luquet, S., & Gangarossa, G. (2024). NAPE-PLD in the ventral tegmental area regulates reward events, feeding and energy homeostasis. Molecular Psychiatry. https://doi.org/10.1038/s41380-024-02427-6
- Roca-Rivada, A., Do Cruzeiro, M., Denis, R. G. P., Zhang, Q., Rouault, C., Rouillé, Y., Launay, J.-M., Cruciani-Guglielmacci, C., Mattot, V., Clément, K., Jockers, R., & Dam, J. (2024). Whole-body deletion of Endospanin protects from obesity-associated deleterious metabolic alterations. JCI Insight. https://doi.org/10.1172/jci.insight.168418
- Montalban, E., Herrera Moro Chao, D., Ansoult, A., Pham, C., Contini, A., Castel, J., Hassouna, R., Hardonk, M., Petitbon, A., Foppen, E., Gangarossa, G., Trifilieff, P., Li, D., Luquet, S., & Martin, C. (2024). Dual role of striatal astrocytes in behavioral flexibility and metabolism in the context of obesity. bioRxiv. https://doi.org/10.1101/2023.03.21.533596
2023
- Leuillier, M., Duflot, T., Ménoret, S., Messaoudi, H., Djerada, Z., Groussard, D., Denis, R. G. P., Chevalier, L., Karoui, A., Panthu, B., Thiébaut, P.-A., Schmitz-Afonso, I., Nobis, S., Campart, C., Henry, T., Sautreuil, C., Luquet, S. H., Beseme, O., Féliu, C., Peyret, H., Nicol, L., Henry, J.-P., Renet, S., Mulder, P., Wan, D., Tesson, L., Heslan, J.-M., Duché, A., Jacques, S., Ziegler, F., Brunel, V., Rautureau, G. J. P., Monteil, C., do Rego, J.-L., do Rego, J.-C., Afonso, C., Hammock, B., Madec, A.-M., Pinet, F., Richard, V., Anegon, I., Guignabert, C., Morisseau, C., & Bellien, J. (2023). CRISPR/Cas9-mediated inactivation of the phosphatase activity of soluble epoxide hydrolase prevents obesity and cardiac ischemic injury. Journal of Advanced Research. https://doi.org/10.1016/j.jare.2022.03.004
- Delbès, A.-S., Quiñones, M., Gobet, C., Castel, J., Denis, R. G. P., Berthelet, J., Weger, B. D., Challet, E., Charpagne, A., Metairon, S., Piccand, J., Kraus, M., Rohde, B. H., Bial, J., Wilson, E. M., Vedin, L.-L., Minniti, M. E., Pedrelli, M., Parini, P., Gachon, F., & Luquet, S. (2023). Mice with humanized livers reveal the role of hepatocyte clocks in rhythmic behavior. Science Advances. https://doi.org/10.1126/sciadv.adf2982
- Montalban, E., Walle, R., Castel, J., Ansoult, A., Hassouna, R., Foppen, E., Fang, X., Hutelin, Z., Mickus, S., Perszyk, E., Petitbon, A., Berthelet, J., Rodrigues-Lima, F., Cebrian-Serrano, A., Gangarossa, G., Martin, C., Trifilieff, P., Bosch-Bouju, C., Small, D. M., & Luquet, S. (2023). The Addiction-Susceptibility TaqIA/Ankk1 Controls Reward and Metabolism Through D2 Receptor-Expressing Neurons. Biological Psychiatry. https://doi.org/10.1016/j.biopsych.2023.02.010
2022
- Bakker, W., Imbernon, M., Gravesen Salinas, C., Herrera Moro Chao, D., Hassouna, R., Morel, C., Martin, C., Leger, C., Denis, R. G. P., Castel, J., Peter, A., Heni, M., Maetzler, W., Nielsen, H. S., Duquenne, M., Schwaninger, M., Lundh, S., Hogendorf, W. F. J., Gangarossa, G., Secher, A., Hecksher-Sørensen, J., Pedersen, T. Å., Prevot, V., & Luquet, S. (2022). Acute changes in systemic glycemia gate access and action of GLP-1R agonist on brain structures controlling energy homeostasis. Cell Reports. https://doi.org/10.1016/j.celrep.2022.111698
- Dumont, C., Li, G., Castel, J., Luquet, S., & Gangarossa, G. (2022). Hindbrain catecholaminergic inputs to the paraventricular thalamus scale feeding and metabolic efficiency in stress-related contexts. The Journal of Physiology. https://doi.org/10.1113/JP282996
- Cruciani-Guglielmacci, C., Meneyrol, K., Denom, J., Kassis, N., Rachdi, L., Makaci, F., Migrenne-Li, S., Daubigney, F., Georgiadou, E., Denis, R. G. P., Sanchez-Archidona, A. R., Paul, J.-L., Thorens, B., Rutter, G. A., Magnan, C., Le Stunff, H., & Janel, N. (2022). Homocysteine metabolism pathway is involved in the control of glucose homeostasis: A cystathionine beta synthase deficiency study in mouse. Cells. https://doi.org/10.3390/cells11111737
- Herrera Moro Chao, D., Kirchner, M. K., Pham, C., Foppen, E., Denis, R. G. P., Castel, J., Morel, C., Montalban, E., Hassouna, R., Bui, L.-C., Renault, J., Mouffle, C., García-Cáceres, C., Tschöp, M. H., Li, D., Martin, C., Stern, J. E., & Luquet, S. (2022). Hypothalamic astrocytes control systemic glucose metabolism and energy balance. Cell Metabolism. https://doi.org/10.1016/j.cmet.2022.09.002
- Berland, C., Castel, J., Terrasi, R., Montalban, E., Foppen, E., Martin, C., Muccioli, G. G., Luquet, S., & Gangarossa, G. (2022). Identification of an endocannabinoid gut-brain vagal mechanism controlling food reward and energy homeostasis. Molecular Psychiatry. https://doi.org/10.1038/s41380-021-01428-z
- Pramfalk, C., Ahmed, O., Pedrelli, M., Minniti, M. E., Luquet, S., Denis, R. G. P., Olin, M., Härdfeldt, J., Vedin, L.-L., Steffensen, K. R., Rydén, M., Hodson, L., Eriksson, M., Parini, P. (2022). Soat2 ties cholesterol metabolism to β-oxidation and glucose tolerance in male mice. Journal of Internal Medicine. https://doi.org/10.1111/joim.13450
- Imbernon, M., Saponaro, C., Cederberg Helms, H. C., Duquenne, M., Fernandois, D., Deligia, E., Denis, R. G. P., Herrera Moro Chao, D., Rasika, S., Staels, B., Pattou, F., Pfrieger, F. W., Brodin, B., Luquet, S., Bonner, C., & Prevot, V. (2022). Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions. Cell Metabolism. https://doi.org/10.1016/j.cmet.2022.06.002
2021
- Prola A., Blondelle J., Vandestienne A., Piquereau J., Denis R.G.P., Guyot S., Chauvin H., Mourier A., Maurer M., Henry C., Khadhraoui N., Gallerne C., Molinié T., Courtin G., Guillaud L., Gressette M., Solgadi A., Dumont F., Castel J., Ternacle J., Demarquoy J., Malgoyre A., Koulmann N., Derumeaux G., Giraud M.-F., Joubert F., Veksler V., Luquet S., Relaix F., Tiret L., Pilot-Storck F. (2021). Cardiolipin content controls mitochondrial coupling and energetic efficiency in muscle. Sci Adv. DOI: https://doi.org/10.1126/sciadv.abd6322.
- Filipe A., Chernorudskiy A., Arbogast S., Varone E., Villar-Quiles R.-N., Pozzer D., Moulin M., Fumagalli S., Cabet E., Dudhal S., De Simoni M.-G., Denis R., Vadrot N., Dill C., Giovarelli M., Szweda L., De Palma C., Pinton P., Giorgi C., Viscomi C., Clementi E., Missiroli S., Boncompagni S., Zito E., Ferreiro A. (2021). Defective endoplasmic reticulum-mitochondria contacts and bioenergetics in SEPN1-related myopathy. Cell Death Differ. DOI: https://doi.org/10.1038/s41418-020-0587-z.
- Dalenberg J.R., Denis R., Luquet S., Small D.M. (2021). Further Evidence that Habitual Consumption of Sucralose with, but Not without, Carbohydrate Alters Glucose Metabolism. Cell Metab. DOI: https://doi.org/10.1016/j.cmet.2021.01.006.
- Cornejo M.P., Denis R.G.P., García Romero G., Fernández G., Reynaldo M., Luquet S., Perello M. (2021). Ghrelin treatment induces rapid and delayed increments of food intake: a heuristic model to explain ghrelin’s orexigenic effects. Cellular and Molecular Life Sciences. DOI: https://doi.org/10.1007/s00018-021-03937-0.
- Tirou L., Russo M., Faure H., Pellegrino G., Demongin C., Daynac M., Sharif A., Amosse J., Le Lay S., Denis R., Luquet S., Taouis M., Benomar Y., Ruat M. (2021). Sonic Hedgehog receptor Patched deficiency in astrocytes enhances glucose metabolism in mice. Mol Metab. DOI: https://doi.org/10.1016/j.molmet.2021.101172.
- Marion C., Zizzari P., Denis R.G.P., Hassouna R., Chebani Y., Leste-Lasserre T., Doat H., Le Pen G., Cota D., Noble F., Luquet S., Pantel J. (2021). The GhsrQ343X allele favors the storage of fat by acting on nutrient partitioning. J Endocrinol. DOI: https://doi.org/10.1530/JOE-20-0576.
dernière mise à jour 09/01/2025
Actualités
Welcome to Alaa Reguei, new PhD student at TPM2PI.
Alaa Reguei will join the TPM2PI team by october 2025, thank to a grant of thee graduate school "Drug Development". His work will focus on "Conception/optimisation of GPCRs biased modulators, combining AI and molecular simulation".
Call for Applications – BFA
The Functional and Adaptive Biology Unit (BFA, Université Paris Cité, CNRS UMR 8251, INSERM ERL 1133, https://bfa.u-pariscite.fr/ ) is launching a call for applications centered on the integrated study of pathological processes related to the nutritional...
InIdex MetaboBrain
Acceptation du projet InIdex MetaboBrain, auquel l'équipe contribuera.

