Endocrinology of Diabetes and Fertility (EnDF)
The endocrine system plays a crucial role in maintaining body homeostasis, and its dysfunction can disrupt essential physiological functions. Research conducted by the EnDF team aims to gain a better understanding of the endocrine systems involved in glucose regulation and reproductive function, in particular the endocrine pancreas and the hypothalamic-pituitary-ovarian (HPO) axis. Our aim is to analyze the physiology of these complex systems, study their interrelationships, and identify environmental or endogenous factors likely to alter their functioning, which can lead to pathologies such as diabetes and infertility.
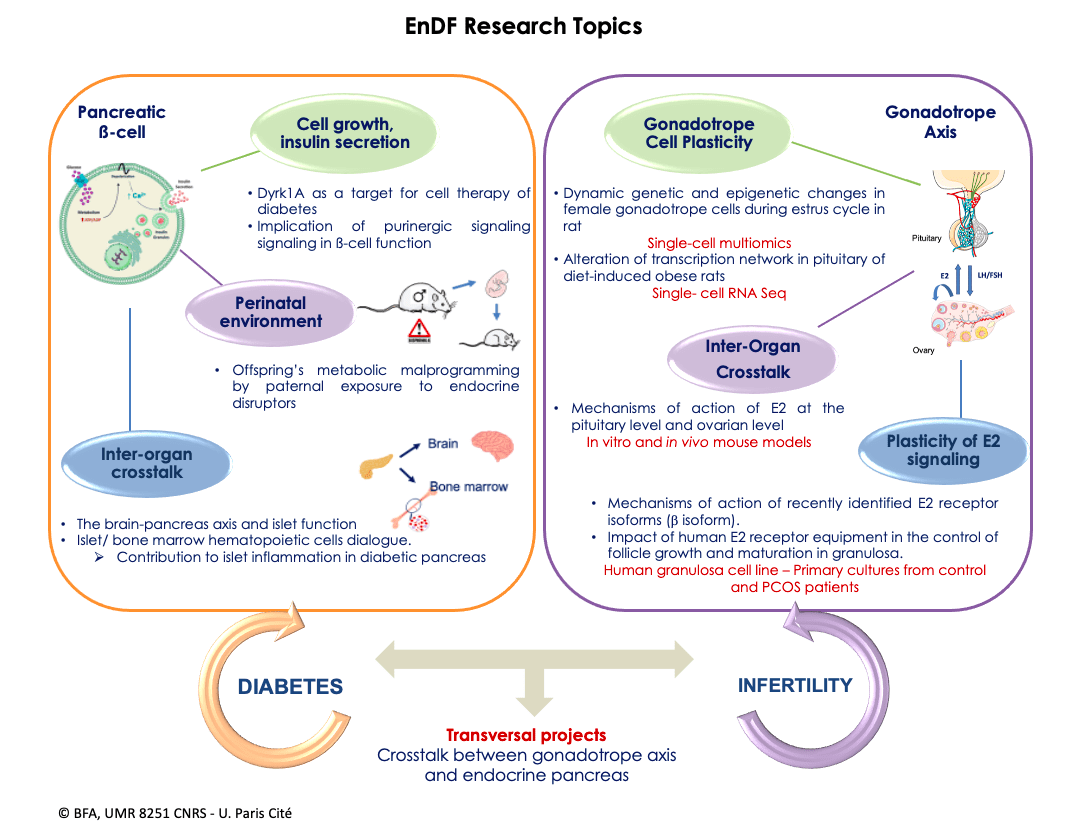
In 2025, the teams Biology and Pathology of the Endocrine Pancreas (B2PE) and Physiology of the Gonadotropic Axis (PAG) merged to create a new team named “Endocrinology of Diabetes and Fertility” (EnDF). The EnDF team is organized into two groups: the first focusing on the endocrine pancreas, the second on the gonadotropic axis. In addition to pursuing projects specific to the 2 groups, the EnDF team works in close collaboration, developing innovative cross-disciplinary projects to study the bidirectional relationship between diabetes and fertility, which has become a major issue in our industrialized societies
Thematic Areas
Endocrine Pancreas Axes
Molecular mechanisms of β-cell growth and function. Physiological and pathological aspects.
- Induction of pancreatic β-cell proliferation and differentiation by modulation of Dyrk1A kinase activity. Convincing studies in human pancreas have demonstrated that there is a significant β-cell mass decreases in advanced type 2 diabetes (T2D). This is due to decreased proliferation and increased apoptosis, probably caused by gluco- and lipotoxicity. This loss of β-cells contributes to inadequate insulin secretion, a central aspect of the development of T2D. To counter this phenomenon, β-cell regeneration and islet preservation are seen as potential therapeutic strategies. In line with our long-lasting activities to identify molecular targets for the stimulation of β-cell regeneration, and in continuation of our previous work on GSK3β (Figeac et al. 2010, Am J Physiol Metab, 2010; Figeac et al. Mol Ther 2012; Pitasi et al. J Endocrinol, 2020), we have focused our research on Dyrk1A, an enzyme in close interaction with GSK3β. In partnership with the biotech company Perha Pharmaceuticals, Dyrk1A inhibitors are being tested in animal models (GK diabetic rats and db/db mice). These studies aim to investigate the effects of inhibitors on β-cell regeneration and identify the signaling pathways involved. The results could lead to the development of new therapies to preserve β-cell function and restore β-cell mass in T2D. Movassat (PI), B. Uzan (PI), S. Tolu, D.Picot. Collaborators: L. Meijer (Perha Pharmaceuticals), J. Dairou (LCBPT, UMR 8601), P. Cattan (Hôp. Saint-Louis, Paris 10). Fundings: Eurostars 2021, ANR PRCE 2022.
- Impact of the microenvironment on the regulation of healthy and diabetic ß-cell function. Several experimental and clinical studies have reported structural and functional alterations in the micro-vascularization of rodent and human islets during diabetic disease. This loss of exchanges between ß-cells and the circulation is thought to be a key factor in ß-cell dysfunction and the loss of regulation of the glucose response that characterizes T2D. One of the glucotoxic consequences of chronic hyperglycemia is excessive ATP production, leading to increased activation of P2-type purinergic signaling. The literature indicates a role for this P2-receptors (P2X) in the deleterious modulation of various tissues (adipose tissue and nerve cells) as well as in retinal and renal micro-vascularization in animal models of T2D. In continuation of our previous work (Quinault et al. Biochem Biophys Acta, 2016; Mesto et al. J Cell Physiol, 2022), our work aims to better understand the possible impact of purinergic signaling affecting ß-cell-endothelial cell interactions in the islet of Langherans in a glucotoxic environment (such as T2D). C. Tourrel-Cuzin (PI), B. Uzan, S. Tolu, J. Movassat, D. Picot.
-
Disrupted hematopoiesis and immune cell infiltration in islets during diabetes. The bone marrow (BM) is the main site of blood cell production and contains hematopoietic stem and progenitor cells (HSPCs). Obesity is known to cause chronic inflammation in adipose tissue, which stimulates HSPC proliferation and increases myeloid cell production. However, the impact of diabetes without obesity on hematopoiesis has not yet been studied. Taking advantage of the Goto-Kakizaki (GK) rat model of T2D, which mimics the β-cell loss and islet inflammation present in diabetic patients, the aim is to characterize hematopoietic phenotypes in the BM and blood of GK rats at different stages of the disease (pre-diabetic and confirmed diabetic). In addition, we aim to understand how diabetes affects hematopoiesis and how a disrupted population of BM cells might influence islet inflammation and β-cell dysfunction in the pancreas (BM transplantations between GK and Wistar (healthy) rats). Subsequently, altered molecular pathways in the hematopoietic populations of GK rats at different stages of the disease will be analyzed to better understand the mechanisms underlying these abnormalities. B. Uzan (PI), C. Tourrel-Cuzin, J. Movassat, D. Picot, S. Tolu.
Environmental programming of metabolic diseases
The general awareness of the impact of the maternal nutritional and toxicological environment on offspring health has grown considerably in our societies in recent decades (Portha et al. Nutrients, 2019). However, the environmental inheritance (non-genetic transmission) of diseases, through the paternal lineage, remains underexplored. We have recently developed new projects on this emerging topic and shown that paternal protein-rich diet alters insulin sensitivity in their offspring in a sex-dependent manner (Gong et al. Biomolecules, 2021). As an extension of our research in this field, we are developing a project to understand the role of the toxicological environment, in response to current societal concerns linked to widespread exposure to environmental pollutants (endocrine disruptors, air pollution). We are studying the impact of paternal pre-conceptional exposure to endocrine disruptors on the metabolic health of offspring. We are studying the profile of small non-coding RNAs in the sperm of male progenitors exposed to BPS, as well as the epigenetic modifications occurring in the pancreatic, muscular, hepatic and adipose tissues of their fetal and adult offspring (Collaboration with Dr. Valérie Grandjean). This study will provide further evidence that changes in the paternal environment prior to conception could have a significant impact on the metabolic health of their offspring in adulthood. J. Movassat (PI), D. L’Hôte, B. Uzan, C. Tourrel-Cuzin, D. Picot, S. Tolu. Collaborations : V. Grandjean (C3M – Inserm U1065). Financement:Société Francophone du Diabète
The link between T2D and neurodegenerative diseases. The islet/brain axis.
Epidemiological data suggest an increased risk of developing Alzheimer’s disease (AD) in individuals with T2D. With the aging of the population, the socio-economic and human burden of these diseases are expected to increase considerably. Despite the large body of epidemiological evidence linking AD and T2D, the molecular mechanisms underlying this association are still unknown. In collaboration with Dr N Janel (BFA, team 1), we recently demonstrated that diabetic GK rats display several circulating Alzheimer’s-like markers (Movassat et al. Front Neurol, 2019). We will now analyze some of the brain and pancreatic features linking T2D to neurodegenerative diseases, in collaboration with Dr. N. Janel and Dr. J. Dairou (LCBPT, UMR 8601). The identification of shared molecular mechanisms between AD and T2D is crucial, as it could lead to the identification of common therapeutic targets for the treatment of these two interconnected diseases. J. Movassat (PI), S. Tolu, B. Uzan, D. Bailbé, C. Tourrel-Cuzin, D. Picot. Collaborators: N. Janel (BFA, Team1), J. Dairou (LCBPT, CNRS UMR 8601).
Gonadotrope Axis
Molecular plasticity underlying the pituitary control of reproductive function.
In mammals, the pituitary integrates multiple signals, locally-produced or emanating from the central nervous system and periphery, to appropriately control gonadal activity during the main phases of reproductive life. The result is high plasticity in the activity of pituitary gonadotrope cell activity, culminating in females during the estrus cycle, characterized by finely-tuned secretion of the two gonadotropins, LH and FSH. Surprisingly, the molecular mechanisms underlying this plasticity are still poorly understood. In addition, pituitary activity is also targeted by nutrition-related signals, notably fatty acids (Garrel et al. Endocrinology 2011; Garrel et al. Endocrinology 2014). Accordingly, we recently reported impaired gonadotropic activity in rats fed a high-fat diet (HFD) in the short or long term. Interestingly, although overfeeding is known to activate inflammatory pathways in several metabolic tissues as well as in the hypothalamus, contributing to metabolic disorders, no increase in gene expression of inflammatory mediators could be detected in the pituitary gland (Garrel et al. Front Endocrinol 2022). L’Hôte (PI), G. Garrel, J. Cohen-Tannoudji. Collaborators: O. Taboureau & N. Cerisier, (BFA, Team 6), Funding: IDEX Emergence grant
Plasticity of estrogen signaling within the gonadotropic axis
E2 plays an essential role in women fertility, by locally regulating ovarian function and by exerting negative and positive feedback on the hypothalamic-pituitary axis. E2 actions are predominantly mediated by the estrogen receptors ERα and ERβ, which induce, after homo- or heterodimerization, genomic and non-genomic signaling. ERβ is mainly expressed in the granulosa cells (GC) of ovarian follicles and contributes to the regulation of follicle growth and maturation. It has very recently been reported that ERβ is expressed, along with ERα, in human pituitary. The main aim of our research project is to identify the roles and signaling mechanisms of ERβ in both organs, which are far from fully understood. Five spliced isoforms of human ERβ have been characterized, termed ERβ1-5. We have recently shown that ERβ1, ERβ2, ERβ4, and ERβ5 are highly expressed in human ovulatory follicle CG, with some but not all isoforms regulating cell growth (Pierre, et al. International Journal of Molecular Sciences, 2021; Chauvin et al. Journal of Molecular Sciences, 2022). Our project is based on three major objectives to decipher: i) how E2 regulates follicle growth and maturation through its various ERβ isoforms, ii) potential alterations in E2 signaling in ovarian disorders such as PCOS, the leading cause of female infertility, and iii) the role of each ER member in controlling pituitary functions. Overall, our project should decipher the specific roles of ERβ isoforms in triggering the various actions of E2 in the human ovary and pituitary, and shed new light on the molecular alterations occurring during infertility. S. Chauvin (PI), L’Hôte and collaborators : Pr Grynberg & Pr Frydman (Hôp. A. Béclère, Clamart); Pr C. Touboul & Dr A. Crestani (Hôp. Tenon, Paris 20); P. Tuffery & S. Murail (BFA, Team 7).
Transversal Axes
Role of estradiol signaling in pancreatic β-cell plasticity
Estradiol (E2) plays a crucial role in glucose homeostasis and energy metabolism, protecting pancreatic islet β-cell function and survival. Accordingly, animal models of T2D show sexual dimorphism with relative protection in females, and clinical data associate menopausal estrogen deficiency with an increased risk of diabetes. Human and rodent β cells express two estradiol receptors (ERa and ERβ). The rapid insulinotropic effect of E2 appears to be mediated primarily by ERβ, while insulin biosynthesis is thought to require the genomic action of ER. However, the molecular mechanisms of E2 action in human βcells remain poorly understood, particularly in relation to diabetes. In this project, we aim to explore the impact of E2 signaling on β-cell growth and function. To this end, we are analyzing the expression of estradiol receptors, in particular human ERβ isoforms, in pancreatic islets from male and female donors (collaboration with Dr. P. Cattan, Hôp. Saint-Louis). Using lentiviral and RNAseq techniques, we are assessing the role of each receptor in E2-stimulated insulin secretion, from β-cells exposed to E2 concentrations reflecting hormonal variations throughout life (puberty, menopause). Finally, the study of estradiol receptor signaling in a diabetic animal model (GK rats) will help to understand how alterations in this signaling could contribute to β-cell degradation and the development of diabetes. Chauvin (PI), C. Tourrel-Cuzin (PI), J. Movassat , S. Tolu. Collaborators: P. Cattan and M. Armanet (Hôp. Saint Louis, Paris 10).
Vulnerability of pituitary and gonadotropic cells to prenatal exposure to a diabetic environment.
The concept of developmental origins of health and disease (DoHAD) describes how perturbations, of the fetal environment, disturb normal epigenetic programming of numerous physiological functions, leading to the development of disease in adulthood. Gestational diabetes (GD) is one example, being associated with an increased risk of metabolic, cardiovascular, neurodevelopmental and reproductive diseases in offspring (Portha et al. Nutrients, 2019). Studies in animal models also show that chronic hyperglycemia can disrupt the epigenome of pituitary gonadotropin cells, affecting reproductive function (Bourgneuf et al. Nat Comm, 2021). The aim of this project is to study, for the first time, the epigenetic vulnerability of pituitary gonadotrope cells in vivo in the context of mild GD, using the GK rat model, which is not solely a T2D model, as it displays features of GDM. L’Hôte (PI), B. Uzan (PI), J. Movassat, J. Cohen-Tannoudji, G. Garrel. Collaborators : C. Racine et N. Diclemente (UMRS_938, Hôp. Saint-Antoine, Paris 12).
Publications
2025
Tolu S, Hamzé R, Moreau M, Bertrand R, Janel N, Movassat J. Beta cell function and global glucose metabolism are impaired in Dp(16)1Yey mouse model of Down syndrome. Diabetes Obes Metab. 2025 Jan 13.
Gautheron G, Péraldi-Roux S, Vaillé J, Belhadj S, Patyra A, Bayle M, Youl E, Omhmmed S, Guyot M, Cros G, Guichou JF, Uzan B, Movassat J, Quignard JF, Neasta J, Oiry C. The flavonoid resokaempferol improves insulin secretion from healthy and dysfunctional pancreatic β-cells. Br J Pharmacol. 2025 Jan;182(1):52-68.
2024
Guillemain G, Khemtemourian L, Brehat J, Morin D, Movassat J, Tourrel-Cuzin C, Lacapere JJ. TSPO in pancreatic beta cells and its possible involvement in type 2 diabetes. Biochimie. 2024 Sep;224:104-113.
Segú H, Jalševac F, Sierra-Cruz M, Feliu F, Movassat J, Rodríguez-Gallego E, Terra X, Pinent M, Ardévol A, Blay MT. Assessing the impact of insect protein sources on intestinal health and disease: insights from human ex vivo and rat in vivo models. Food Funct. 2024 Apr 22;15(8):4552-4563.
Le Ciclé C, Pacini V, Rama N, Tauszig-Delamasure S, Airaud É, Petit F, de Beco S, Cohen-Tannoudji J, L’Hôte D. Migration des cellules gonadotropes durant l’embryogenèse hypophysaire – Rôle des facteurs de transcription NEUROD1 et NEUROD4 [Transcription factors NEUROD1 and NEUROD4 in the migration of gonadotrope cells during pituitary embryogenesis]. Med Sci (Paris). 2024 Nov;40(11):810-812.
More
No Results Found
The page you requested could not be found. Try refining your search, or use the navigation above to locate the post.
