Degeneration, Neurotransmission, Stress and Aging
Team leaders: Serge Birman (CNRS), Véronique Monnier (UPCité)
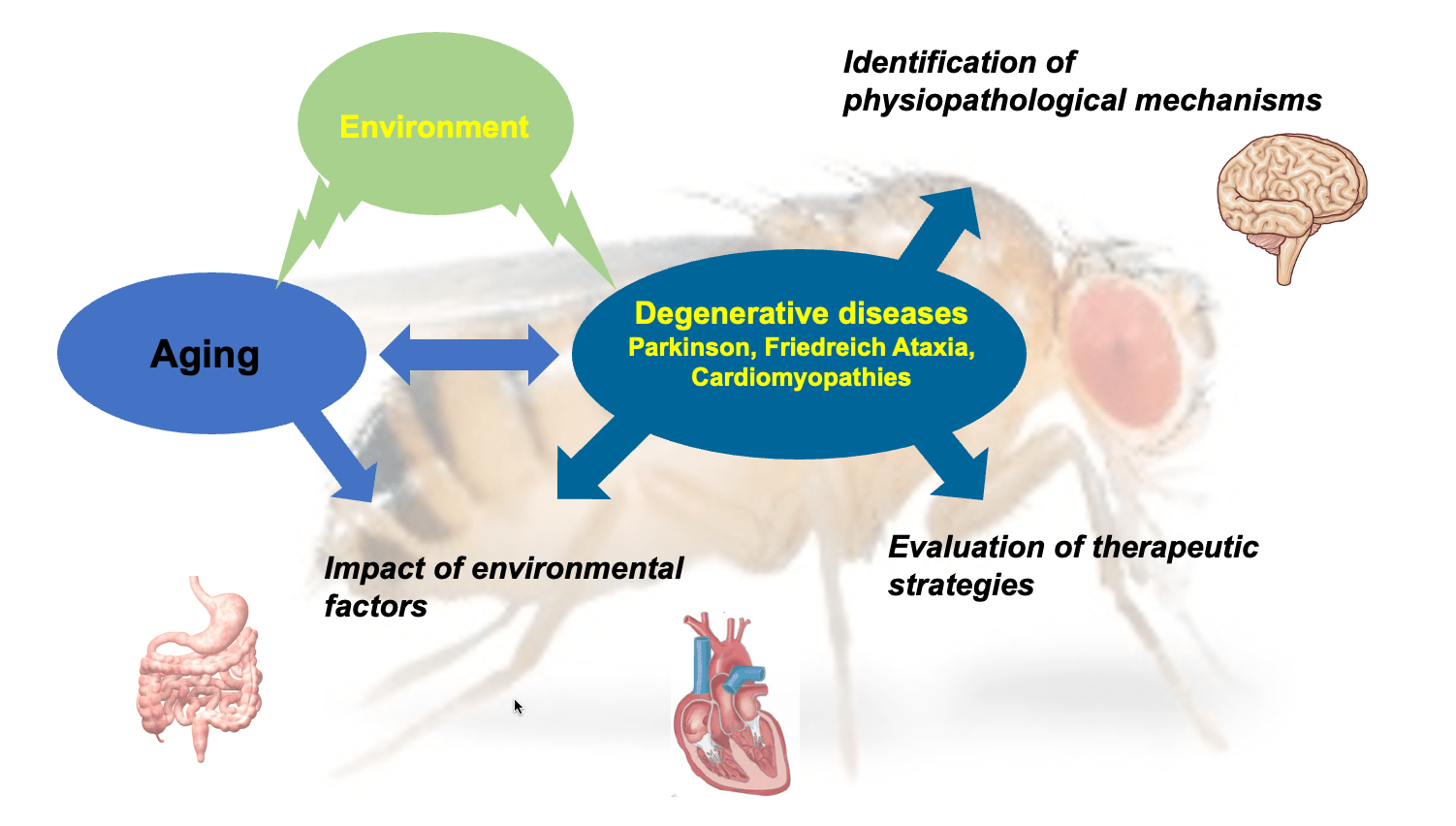
The major objective of our team is to study the genetic, cellular and synaptic processes involved in human aging and degenerative disorders. We aim to identify and characterize the mechanisms and the genes that are involved, to assess the impact of environmental factors (such as diet or pesticides) and on this basis to identify new therapeutic interventions. We use Drosophila as a model organism, enabling us to develop integrative multi-scale and in vivo approaches. Our research focuses in particular on two neurodegenerative diseases: Friedreich’s ataxia and Parkinson’s disease. In both cases, the main feature is neurodegeneration, but other organs are also affected, such as the intestine for Parkinson’s disease, and the heart for Friedreich’s ataxia.
© Copyright of the BFA Unit
Axes
Axis 1: Physiopathology of neurodegenerative diseases and cardiomyopathies
Our first research axis focuses on genetic and cellular aspects of degeneration affecting the nervous and cardiac systems. For Parkinson’s disease, we are focusing on several genes already known to be involved in familial and sporadic forms of the disease (specifically SNCA, which codes for α-synuclein, DJ-1and LRRK2), with the aim of better understanding how these genes are involved in disease progression and the degeneration of dopaminergic neurons. In Friedreich’s ataxia, the disease is caused by a decrease in the expression of frataxin, a mitochondrial protein involved in the biosynthesis of iron-sulfur clusters (ISCs). We are studying how frataxin deficiency impacts this essential mitochondrial process, leading to multiple cellular dysfunctions. In the case of cardiomyopathies, our aim is to use Drosophila as a tool for validating new genes and mechanisms, through functional screening of candidate genes identified in patients.
Axis 2: Evaluation of therapeutic approaches
This axis is dedicated to the identification and in vivo evaluation of therapeutic approaches in Drosophila models of Parkinson’s disease and Friedreich’s ataxia. We are essentially developing two types of approaches:
1) the pharmacological screening of candidate molecules, which are either selected for their known effects on processes affected in the diseases (such as oxidative stress, mitochondrial dysfunction or perturbation of iron homeostasis…) or molecules derived from high-throughput screening in vitro or in silico carried out by our collaborators
2) the research and evaluation of therapeutic peptides. In particular, we are looking for interfering peptides that can destabilize protein-protein interactions involved in physiopathological processes. This area benefits from the expertise of the TMP2I team of the unit.
Axis 3: Impact of environmental factors on aging and degeneration
Environmental conditions can have a deep impact on longevity and age-related neurodegenerative diseases. We are looking at this from two perspectives:
1) the long-term impact, maintained over several generations i.e transgenerational, that variations in environmental factors can have on physiological aging.
2) the effects of environmental exposure to neurotoxic compounds. An increased risk of developing Parkinson’s disease has been observed in people, like farmers, who are exposed for a long period of time to pesticides. Drosophila is a model organism particularly relevant to study the molecular link between pesticide exposure and Parkinson’s disease.
Recent publications
Petitgas, C., Seugnet, L., Dulac, A., Matassi G., Mteyrek, A., Fima, R., Strehaiano, M., Dagorret, J., Chérif-Zahar, B., Marie, S., Ceballos-Picot, I., Birman, S. (2024) Metabolic and neurobehavioral disturbances induced by purine recycling deficiency in Drosophila. eLife 12:RP88510. doi:10.7554/eLife.88510.3
Jullian E, Russi M, Turki E, Bouvelot M, Tixier L, Middendorp S, Martin E, Monnier V. Glial overexpression of Tspo extends lifespan and protects against frataxin deficiency in Drosophila. (2024) Biochimie. doi: 10.1016/j.biochi.2024.05.003
Mathas, N., Poncet, G., Laurent, C., Larigot, L., Le-Grand, B., Gonis, E., Birman, S., Galardon, E., Sari, M.-A., Tiouajni, M., Nioche, P., Barouki, R., Coumoul, X., Mansuy, D., Dairou, J. (2023) Inhibition by pesticides of the DJ-1/Park7 protein related to Parkinson disease. Toxicology 487:153467. doi:10.1016/j.tox.2023.153467
Rahmani, Z., Surabhi, S., Rojo-Cortés, F., Dulac, A., Jenny, A., Birman, S. (2022) Lamp1 deficiency enhances sensitivity to α-synuclein and oxidative stress in Drosophila models of Parkinson disease. Int. J. Mol. Sci. 23(21), 13078. doi:10.3390/ijms232113078
Ader F, Russi M, Tixier-Cardoso L, Jullian E, Martin E, Richard P, Villard E, Monnier V. Drosophila CRISPR/Cas9 mutants as tools to analyse cardiac filamin function and pathogenicity of human FLNC variants. (2022) Biol Open. bio059376. doi: 10.1242/bio.059376.
Dulac, A., Issa, A.-R., Sun, J., Matassi, G., Jonas, C., Rahmani, Z., Chérif-Zahar, B., Cattaert, D., Birman, S. (2021) A novel neuron-specific regulator of the V-ATPase in Drosophila. eNeuro 8(5):ENEURO.0193-21.2021. doi:10.1523/ENEURO.0193-21.2021
Martin E, Heidari R, Monnier V, Tricoire H. Genetic screen in adult Drosophila reveals that dCBP depletion in glial cells mitigates Huntington disease pathology through a Foxo-dependent pathway. (2021) Int J Mol Sci. 22(8):3884. doi: 10.3390/ijms22083884
Teseo S, Houot B, Yang K, Monnier V, Liu G, Tricoire H. G. sinense and P. notoginseng extracts improve healthspan of aging flies and provide protection in a Huntington disease model. (2021) Aging Dis. 12(2):425-440. doi: 10.14336/AD.2020.0714-1.
Actualités de l’équipe
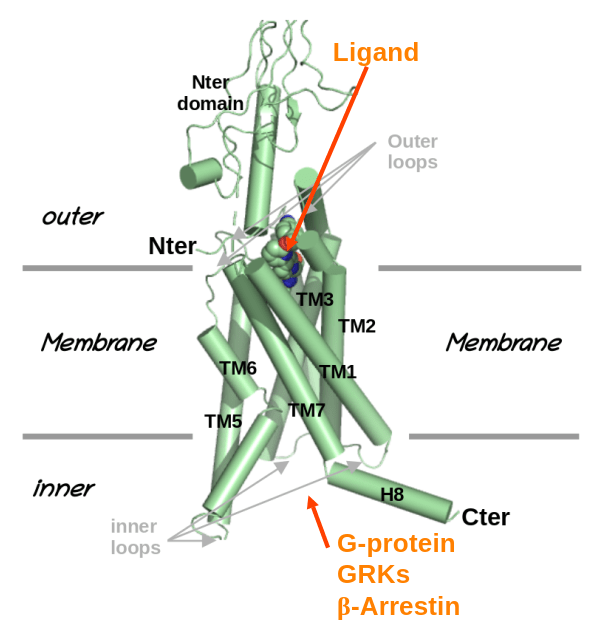
Welcome to Alaa Reguei, new PhD student at TPM2PI.
Alaa Reguei will join the TPM2PI team by october 2025, thank to a grant of thee graduate school "Drug Development". His work will focus on "Conception/optimisation of GPCRs biased modulators, combining AI and molecular simulation".
Call for Applications – BFA
The Functional and Adaptive Biology Unit (BFA, Université Paris Cité, CNRS UMR 8251, INSERM ERL 1133, https://bfa.u-pariscite.fr/ ) is launching a call for applications centered on the integrated study of pathological processes related to the nutritional...
InIdex MetaboBrain
Acceptation du projet InIdex MetaboBrain, auquel l'équipe contribuera.
