Central Control of Feeding behaviour and Energy Expenditure
The core approach of our team Central COntrol oF Feeding behaviour and Energy Expenditure (C2OFFEE) is to leverage the power of modern molecular genetic tools in integrated approaches in order to dissect in vivo the physiological, cellular and molecular basis by which the dialogue between the brain and peripheral organs result in adaptive regulation of the various parameters of energy balance notably in its rewarding, motivational and sensory components, metabolic efficiency, allostatic response.

C2OFFEE team research topics
© BFA, UMR 8251 CNRS – U Paris Cité
Research axes
Molecular determinants of brain susceptibility/resilience to feeding & metabolic diseases

Our recent studies show that circulating triglycerides (TG) can be metabolized in the dopaminergic (DA) reward system and modulate reward-associated behaviors by modulating the activity of neurons expressing the DA receptor type 2 (D2R) (Berland et al, 2020, Cell Metab 31, 773-790 e711. ; Berland et al., 2021, Trends Endocrinol Metab 32, 693-705; Cansell et al, 2014, Molecular psychiatry 19, 1095-1105). Furthermore, in humans, we observed that postprandial TG excursion correlated with brain activities triggered by food cues. Furthermore, we observed that in humans, the modulatory effect of TGs on the central response was dependent on the integrity of D2R signaling, and influenced by the TaqIA/Ankk1 polymorphism (a widespread mutation associated with deregulation of D2R signaling). These findings unveil a novel mechanism by which dietary TGs directly modify signaling events, providing a new mechanistic basis by which energy-rich diets lead to addictive/compulsive-like behaviors (Berland et al., 2020, Cell Metab 31, 773-790 e711.; Sun et al., 2017, Trends Cogn Sci 21, 372-384).
TaqIA/Ankk1 polymorphism and reward dysfunction
The TaqIA A1 polymorphism (SNP, rs1800497) is located in the gene encoding ANKK1 (ankyrin repeat and kinase domain containing 1 kinase) near the Drd2 gene. The TaqI SNP is the most studied polymorphism in psychiatric disorders, but apart from correlative studies in humans, there is no described mechanism for the role of ANKK1 (for review see (Sun et al., 2017, Trends Cogn Sci 21, 372-384)).
We developed an Ankk1fl/fl mouse line and investigated the consequences of Ankk1 ablation in reward circuits. We found that Ankk1 controls reward and metabolism by regulating the activity of D2R-expressing neurons (Montalban. E et al. 2023. Bio Psy). To date, we have also developed a mouse model in which the Ankk1 mutation has been introduced by CRISPR/Cas9. This model allows us to identify potential molecular mechanisms by which the A1 and A2 variants of Ankk1 might affect neuronal activity in the reward network.
Interoceptive dynamics controlling homeostatic and reward processes
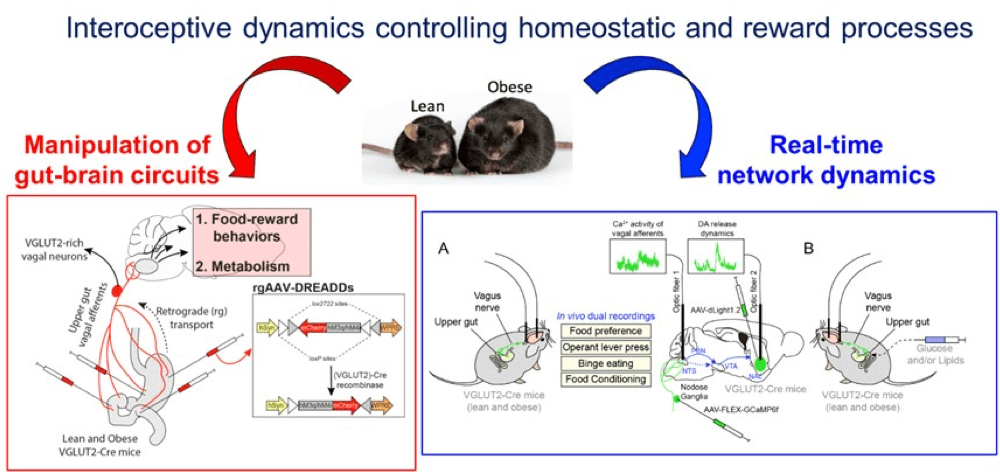
Interoceptive signals from the periphery, via the vagus nerve, constantly inform the brain of the physiological states of peripheral organs, thereby regulating the activity of brain circuits and influencing the development of adaptive strategies orchestrated by the brain. Recently, we demonstrated that ambient peripheral endocannabinoids (eCBs) bidirectionally regulate the activity of the gut-brain vagal axis (Berland et al., 2022, Mol Psychiatry 27, 2340-2354.) and contribute to the establishment of reward-related eating disorders. Furthermore, we have also discovered that interoceptive and homeostatic information can be relayed between downstream brain regions (brainstem) and upstream brain regions by mobilising the extensive network of the paraventricular thalamus (PVT) (Dumont et al., 2022 JPhysiol). Our current hypothesis is based on the idea that maladaptive plasticity of the vagus nerve and distortion of the interoceptive trajectory may lead to dysregulation of the neural centres of the ‘extended’ gut-brain axis and promote eating disorders (i.e. obesity).
Firstly, we will assess whether and how obesity functionally disrupts the interconnected neural networks and dynamics that enable the integration of interoceptive information and the development of (maladaptive) responses. We will mainly study new intestinal-brain interoceptive trajectories which, by converging on different networks, lead to the regulation of food valence (i.e. reinforcement, reward), in particular the vagus nerve-brain stem-NAc pathway and its intermediate centre the PVT. Using projection- and cell-type-specific approaches, we will use fibre photometry to simultaneously record in vivo Ca2+ activity and the release dynamics of mediators (dopamine, acetylcholine, GLP-1, glutamate) in several segments of the gut-brain axis. Interoceptive trajectories will be studied during spontaneous/provoked feeding, food preference, food-motivated behaviour and intragastric nutrient administration in control and obese mice. We will then use microinjections restricted to interconnected networks of Cre/Flp-dependent viruses expressing modified receptors (Gq, Gi, NaChBac, Kir2.1) to manipulate the neuronal components of the gut-brain axis in the processes of food reward, energy metabolism and weight gain. Once again, our functional analysis will focus on the vagus nerve-brain trunk-NAc network and its intermediate centre the PVT. Finally, at the molecular level, we will explore how the obesogenic environment leads to transcriptomic (mis)adaptations of the neuronal networks making up the ‘extended’ gut-brain axis, using projection- and/or cell subtype-specific methodologies.
Impact of metabolic state on olfaction and multisensory signaling

Food intake is a highly multisensory experience, combining smell, taste and touch. Odour, one of the most relevant sensory cues for predicting the presence of food, is likely to play a key role in food choice and consumption. Several studies have shown that the olfactory system is at the crossroads between sensory processing and metabolic detection (Faour et al., 2022, Neuropharmacology 206, 108923). The olfactory bulb detects and is modulated by the main hormones and nutrients involved in regulating food intake. However, we still do not know the impact of the brain region that exerts major control over food intake, the hypothalamus, on olfactory activity and olfaction-induced eating behaviour. Our hypothesis is that the hypothalamo-olfactory network modulates the quality and/or reward value of an odorant as a function of internal state. We combine behavioural and genetic approaches (transgenic mice, Designer Receptors Exclusively Activated By Designer Drugs) and fluorescent biosensor imaging (GCaMP-based fibre photometry) in behaving mice to assess the impact of manipulating AgRP neurons or the impact of trigeminal nerve inhibition on olfactory bulb neuronal activity and olfactory feeding behaviour.
In addition, most odorants also activate the trigeminal nerve, which is part of the somatosensory pathway. It is currently unclear whether functional changes in trigeminal nerve activation could be involved in impaired olfaction in obese individuals. We are studying the interactions between olfaction and the trigeminal system.
DOLFINA project: Restoring the sense of smell in anosmic patients.
Astrocytic control of metabolism and reactivity in response to obesity
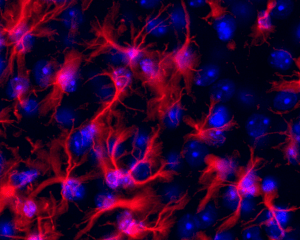 Astrocytes in the hypothalamus by immunofluorescence (© BFA, UMR 8251 CNRS – U Paris Cité)
Astrocytes in the hypothalamus by immunofluorescence (© BFA, UMR 8251 CNRS – U Paris Cité)
Nutritional overload and obesity lead to an adaptive response in astrocyte physiology, resulting in altered central control of food intake, energy expenditure and glucose metabolism (Garcia-Caceres et al., 2019, Nature neuroscience 22, 7-14).
We characterised alterations in the structural and functional properties of astrocytes and astrocyte-neuron communication and found that hypothalamic astrocytes in the paraventricular nucleus exert direct control over body weight regulation and glucose metabolism (Herrera Moro Chao et al., 2022 Cell Metab 34, 1532-1547 e1536).
In the striatum, we have shown that activation of astrocytes in the dorsal striatum rescues obesity-induced cognitive deficits and restores striatal neural synchrony. Furthermore, activation of astrocytes in the nucleus accumbens alters substrate use and whole-body energy expenditure (Montalban et al 2023, BioRxiv https://www.biorxiv.org/content/10.1101/2023.03.21.533596v1).
We want to go further and investigate the cellular and molecular profile of astrocytes in the adaptive response to IOD. We are investigating the impact of astrocyte-astrocyte and astrocyte-neuron communication in vivo using pharmacological and genetic tools, including DREADD/chemiogenetic manipulation of Gq/Gi signalling. These tools will be coupled with in vivo recording of metabolic efficiency and astrocyte and neuronal activity (fibre photometry / GCaMP).
Recent publications
- Onimus O, Arrivet F, Le Borgne T, Perez S, Castel J, Ansoult A, Bertrand B, Mashhour N, de Almeida C, Bui LC, Vandecasteele M, Luquet S, Venance L, Heck N, Marti F, Gangarossa G. The gut-brain vagal axis governs mesolimbic dopamine dynamics and reward events. Sci Adv. 2026 Jan 30;12(5):eadz0828. doi: 10.1126/sciadv.adz0828.
- Montalban E, Ansoult A, Herrera Moro Chao D, Pham C, Franco C, Contini A, Castel J, Hassouna R, Hardonk MH, Petitbon A, Foppen E, Gangarossa G, Trifilieff P, Li D, Luquet S, Martin C. Striatal astrocytes modulate behavioral flexibility and whole-body metabolism in mice. Nat Commun. 2025 Jul 7;16(1):5417. doi: 10.1038/s41467-025-60968-y.
- Huwart SJP, Fayt C, Gangarossa G, Luquet S, Cani PD, Everard A. TLR4-dependent neuroinflammation mediates LPS-driven food-reward alterations during high-fat exposure. J Neuroinflammation. 2024 Nov 23;21(1):305. doi: 10.1186/s12974-024-03297-z.
- Henderson F, Dumas S, Gangarossa G, Bernard V, Pujol M, Poirel O, Pietrancosta N, El Mestikawy S, Daumas S, Fabre V. Regulation of stress-induced sleep perturbations by dorsal raphe VGLUT3 neurons in male mice. Cell Rep. 2024 Jul 23;43(7):114411.
- Onimus, O., Arrivet, F., Souza, I.N.O., Bertrand, B., Castel, J., Luquet, S., Mothet, J.P., Heck, N., and Gangarossa, G.. The gut-brain vagal axis scales hippocampal memory processes and plasticity. Neurobiol Dis 2024 199, 106569.
- Castel J, Li G, Onimus O, Leishman E, Cani PD, Bradshaw H, Mackie K, Everard A, Luquet S, Gangarossa G. NAPE-PLD in the ventral tegmental area regulates reward events, feeding and energy homeostasis. Mol Psychiatry. 2024 Feb 15.
More
Liver cells control our biological clock
Scientists have discovered that the liver influences the biological clocks of other organs.
last update 15/01/2025
News
No Results Found
The page you requested could not be found. Try refining your search, or use the navigation above to locate the post.




